Big Pharma prefers medicines with a defined mode of action (MoA). It eases regulatory approval, reimbursement by public insurance, marketing, and communication with the physicians. Side effects are accepted as collateral damages. Studies for the approval of new topical eye drops frequently compare the new drug with its vehicle (i.e. the drug formulation without the active ingredient API). This strategy allows eliminating vehicle associated side effects. In contrast to the MoA approach I personally favor a more holistic approach to medicine (see my anecdote). In this Chapter I therefore try to provide an introduction to the complex interactions within the ocular surface. Later Chapters will expand to interactions between other parts of the human body and the ocular surface. continue
The term homeostasis has its roots in the concept of constancy of the internal body environment described by the French physiologist Claude Bernard who on 21 August 1865 presented seven volumes of his published lectures to the Académie des Sciences [1]. Walter Cannon generalized Bernard’s theory taking into consideration the numberless disturbances the body is subjected to in an open system and the necessary regulatory and control activities for keeping balance. Cannon wrote ‘The constant conditions which are maintained in the body might be termed equilibria. That word, however, has come to have fairly exact meaning as applied to relatively simple physico-chemical states, in closed systems, where known forces are balanced. The coordinated physiological processes which maintain most of the steady states in the organisms are so complex and so peculiar to living beings – involving, as they may, the brain and nerves, the heart, lungs, kidneys and spleen, all working cooperatively – that I have suggested a special designation for these states, homeostasis. The word does not imply something set and immobile, a stagnation. It means a condition – a condition which may vary, but which is relatively constant’ [2]. In fact any physiological attribute will fluctuate between minimum and maximum ‘normal’ values and is kept in balance using complex feedback mechanisms. For essential physiological functions the body has developed multiple regulatory pathways so that even if one pathway fails the function will still be kept in balance. In 1932 Cannon published his book ‘The Wisdom of the Body’ describing homeostatic regulation for the general reader. Prolonged exposure to non-damaging changes in the environmental conditions may lead to transient modifications of the homeostatic range, for example an adaptation to the life in higher altitudes with reduced oxygen supply. Kelvin Davies proposed the term ‘adaptive homeostasis’ for such transient modifications [2]. 40 years earlier Hans Selye had proposed the term ‘heterostasis’ for the transition to a new steady state triggered by extended exposure to exogenous stimulation [3].
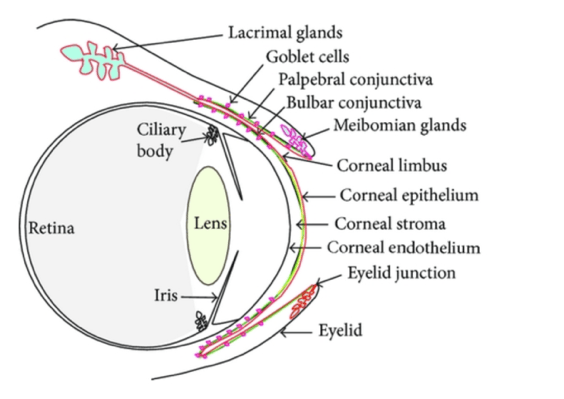
Figure 1: The ocular surface (from: http://dx.doi.org/10.1155/2013/103947)
The ocular surface (OS) is an organ with complex interaction and defense mechanisms. It comprises the cornea, conjunctiva, eyelids, eye lashes, tear film, main and accessory lacrimal glands, and the Meibomian glands [4]. Homeostasis of the ocular surface is the result of an equilibrium regulated by a plethora of interacting entities (cells, extracellular matrix, nerves, oxygen supply, hormones, cytokines …). As a reaction to external stimuli this system will temporarily shift its equilibrium, but return to homeostasis once the stimulus is absent. Any major disturbance of this complex system over a prolonged period of time may result in ocular surface disease (OSD). While initially reversible, a persisting challenge of OS homeostasis will eventually evolve into a chronic status of self-sustaining disease (heterostasis), frequently termed vicious circle in the ophthalmic literature. One of these multifactorial surface disorders has been named dry eye disease (DED) and has been defined as ‘a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles’ [5]. The concept of ‘loss of homeostasis’ of the tear film acknowledges the possibility that many different changes can occur in response to one or more of the underlying causes of dry eye [4]. There is a multitude of ocular surface conditions, some of which closely mimic or mask DED, and may occur concurrently with DED [6]. For instance the eye and its surrounding tissues are commonly involved in local and systemic immunological hypersensitivity reactions [7]. Ocular allergic disorders include seasonal allergic conjunctivitis (SAC), perennial allergic conjunctivitis (PAC), vernal keratoconjunctivitis (VKC), giant papillary conjunctivitis (GPC) and atopic keratoconjunctivitis (AKC). In dermatology it is well established that localized allergic reactions and atopy are associated with epidermal barrier dysfunction [8-11]. Likewise ocular allergy and atopy appear to be associated with compromised conjunctival and corneal epithelial barrier function [12-14]. Ocular allergic disorders are frequently associated with itching eyes and eye rubbing. It is therefore not surprising that atopy and eye rubbing are risk factors for keratoconus development [15-19]. Over decades, keratoconus was considered a non-inflammatory corneal disorder. Recently there is growing evidence that keratoconus has a significant inflammatory component [19-25]. It seems, therefore, worthwhile to try a holistic approach into ocular surface homeostasis and disease.
Let us first have a closer look at the current paradigm of DED as elaborated by the Tear Film and Ocular Surface Society Dry Eye WorkShop II (TFOS DEWS II) and published in 2017, and its focus on the homeostasis of the tear film. The tear film plays a vital role in providing lubrication and protection of the ocular surface, as well as maintaining a smooth refractive surface for optimal visual performance [4]. The preocular tearfilm overlies the external surfaces of conjunctiva and cornea [26]. It consists of a superficial lipid layer on top of a mucoaqueous layer which interacts directly with the glycocalyx of the epithelium.
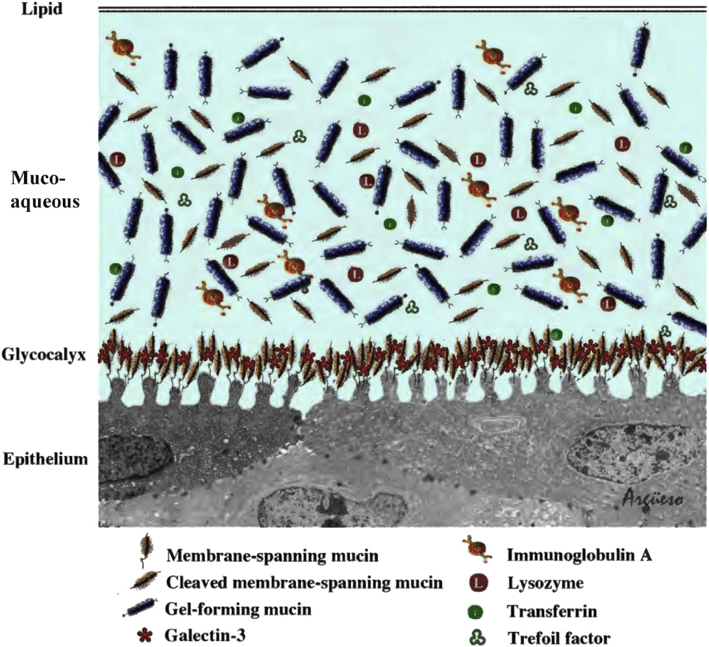
Figure 2: Tear film structure (from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6035753/pdf/nihms978473.pdf; originally published in [43])
The lipid layer derives from the Meibomian gland secretion at the lid margins and plays a significant role in stabilizing the tear film [27]. The DEWS II report distinguishes between two major etiologies of DED: aqueous deficient and evaporative dry eye and names Meibomian gland disease (MGD) a contributor to evaporative dry eye and leading cause of dry eye [4]. We are owing the believe in tear hyperosmolarity as a driving factor in DED to the enormous empathy of Jeffrey Gilbard [28]. Recently tear hyperosmolarity in association with MGD has been suggested as the major driving force in the vicious circle of chronic ocular surface inflammation [29,30]. On the other hand homeostasis of osmolarity is one of the major regulatory issues of the entire human body [31]. The innervation of the corneal epithelium includes cold thermoreceptors sensitive to changes in temperature and osmolarity [32]. Increases in extracellular osmolarity or temperature decreases of the tear film regulate the eye blinking [33]. This in turn results in redistribution of tear film from the tear reservoir of the tear meniscus. Tear film osmolarity measurements usually take samples from the lower tear meniscus. There is growing evidence that the average tear osmolarity measured in patients with DED is not significantly different from healthy tears [34,35]. We may, therefore, assume that the preocular tear film is in the majority of DED patients immediately after blinking similar to the one in normal eyes. During the intervals between blinking the osmolarity will gradually increase due to tear evaporation. The thickness of the tear lipid layer has only negligible influence on the tear evaporation rate [36-38]. Therefore, Meibomian gland disease (MGD) has virtually no direct influence on the tear evaporation rate. The prevailing belief that the tear film in DED is compromised due to increased evaporation as a consequence of a defective lipid layer in not supported by scientific evidence [39]. ‘Evidence from literature is indicating that the role of the tear film lipid layer is to enable the formation of a thin film, to prevent collapse of this thin film and to form a strong barrier that forces the aqueous to flow to the puncta rather than being squeezed over the eyelids during a blink’ [39]. The reduced blinking rate caused by extensive viewing of digital device screens can result in more tear evaporation between blinks and thus in greater fluctuations of osmolarity in the precorneal tear film between blinks, at different times of the day, and from day to day [40,41]. Prolonged periods of precorneal tear film hyperosmolarity trigger inflammation of the ocular surface [29]. This needs to be taken into consideration particularly in the diagnosis and therapy of the increasing number of young patients suffering from dry eye symptoms. It must be the foremost therapeutic target to prevent these patients from developing chronic ocular surface inflammation.
Aqueous DED is where the term ‘dry eye’ originated from. This term was used since the 1950s originally for Sjögren syndrome patients with ocular involvement. The healthy ocular surface epithelium is topographically smooth, lined with an antiadhesive, water binding, protective glycocalyx mainly consisting of membrane bound mucins (see Figure 2). It is covered by a mucoaqueous tear film with water binding, lubricating properties predominantly due to dissolved gel-forming mucin MUC5AC secreted by conjunctival goblet cells [42-44]. The glycoprotein MUC5AC consists of a long protein molecule with shorter oligosaccharide side chains. The flow behavior of dissolved long linear molecules (polymers) is determined by their ability to entangle [45]. Such polymer solutions exhibit viscoelastic, shear thinning characteristics as reported by Kaura and Tiffany for the tear film [46-49]. The tear film requires a high viscosity at rest (zero shear viscosity ƞ0) for stability reasons and a low viscosity during blinking to prevent excess shear stress on the ocular surface epithelia. Tiffany considered viscosity to be a physical property of bulk tear fluid. The recent understanding of a MUC5AC gradient within the mucoaqueous tear layer with higher MUC5AC concentration next to the glycocalyx of the apical epithelial cells requires a reconsideration of the flow behavior or tears. ƞ0 is a function of the product of concentration (c) and chain length (Mw) of the dissolved polymer. At constant chain length and shape (average molecular weight and glycosylation) of the dissolved MUC5AC the zero shear viscosity ƞ0 will only depend on the MUC5AC concentration c.
ƞ0 ~ ck
For an ideal solution of entangled polymer chains the exponent is k = 1 for dilute solutions and k = 3.4 for semidilute solutions [50,51]. Therefore, even minor variations of MUC5AC concentration will strongly influence the viscosity at rest ƞ0 of the mucoaqueous layer next to the glycocalyx of the epithelia. This also explains the formation of mucous strands as a result of excess MUC5AC secretion. Dry eye conditions are changing the rheology of tears [47]. Every form of dry eye, but also atopic keratoconjunctivitis are associated with ocular inflammation, loss of Goblet cells and reduced levels of MUC5AC [43,52-57]. The decreases in concentration, glycosylation or molecular weight of MUC5AC are associated with a disproportionate loss in lubricating efficacy of the tear film. Elevated friction between the cellular structures of the ocular surface has been recognized as the driving force in all forms of OSD, not only DED [57]. Increased friction between the ocular surface tissues can not only originate from inadequate MUC5AC quantities dissolved in the mucoaqueuos tear layer, but also from a compromised glycocalyx of the apical epithelial cells or from topographical irregularities of the ocular surface [58]. The concentration of MUC5AC in the aqueous layer may either be too high causing mucous strands, blurring and high friction during blinking or too low resulting in insufficient lubrication and instable tear film [59]. In order to preserve homeostasis of the tear film the eye may initially try to compensate aqueous deficient tears by down-regulating the MUC5AC secretion in order to keep the flow characteristics of the tears constant. This assumption is consistent with the observation of Argüeso and colleagues who reported a significantly lower number of RNA transcripts for the goblet cell specific MUC5AC in the tear fluid of patients with Sjögren syndrome [53]. Dogru and colleagues reported in patients with atopic keratoconjunctivitis a significantly reduced MUC5AC synthesis in combination with upregulated synthesis of membrane bound mucins presumably to protect the surface from mechanical damage [54,55]. Any persisting elevated friction will result in epithelial damage and may be visible as corneal and conjunctival staining and lid wiper epitheliopathy, inflammation of the lid rim eventually associated with obstruction of Meibomian gland orifices, and/or changes of ocular surface topography, like lid-parallel conjunctival folds (LIPCOF) or sandbank epitheliopathy [60-62]. Elevated friction and shear stress between tissues during blinking exercises stress on the architecture of the epithelial tissues including nerves. As little as 60 seconds of eye rubbing have been shown to cause elevated levels of inflammatory markers like MMP-13, IL-6 and TNF-α in the tear film of healthy eyes [13]. It may therefore be assumed that permanent stress on the ocular epithelial tissues from elevated friction will trigger inflammation and finally initiate chronic inflammation. Van Setten coined the term attrition for the mechanical stress exercised to epithelial tissues by friction, and identified attrition as a potential cause for ophthalmic surgical failure [63]. None of the current diagnostic methods available in ophthalmological routine practice provide a measure of the rheology of the tear film. Schirmer 1 test and tear meniscus volume provide an idea on the production rate of aqueous tears. It would be desirable to be able to simultaneously assess the MUC5AC secretion rate of the conjunctival goblet cells in ophthalmic routine practice to enable the judgement of the tear rheology as an important diagnostic parameter. The measurement of tear film break-up time (BUT) and break-up pattern provide information on tear film stability as a combination of the viscoelastic quality of the mucoaqueous tear layer, the water binding capacity of the glycocalyx, localized ocular surface irregularities and the quality of the lipid layer [64]. From a therapeutic point of view it is important first of all to identify and if possible diminish the underlying cause of increased friction, and if this is impossible, to improve the ocular surface lubrication by the consequent use of topical non-preserved lubricant eye drops in order to prevent the transition into chronic disease (heterostasis).
The ocular surface is directly exposed to the outside environment, and is subject to desiccation and interaction with pathogens and allergens. Mechanical, anatomical and immunological defense mechanisms protect the outer eye [65]. The tear film plays an important role by continuously washing noxious agents out of the ocular surface [14]. The epithelium that covers the ocular surface is of stratified squamous nonkeratinizing type, thus subject to continuous renewal [43]. The tear film contacting membrane of the apical epithelial cells of both cornea and conjunctiva has ridge-like folds, termed microplicae, that form regular ondulations in cross sections. These cells express and produce the thick glycocalyx of transmembrane mucins (see Figure 2). The functions of these very robust membrane-tethered mucins at the ocular surface are: water-binding, disadhesion, prevention that eyelid conjunctival epithelium adheres to corneal epithelium, lubrication, and barrier effect; indeed, corneal and conjunctival epithelia are the first line of defense against the entrance of noxious agents. The glycocalyx forms the transcellular barrier while the intercellular junctions between epithelial cells play a paracellular barrier role [14]. The foremost apical cell layer of the epithelium contains tight junctions that appear as a series of focal connections between adjacent cells, sealing the intercellular space. Desmosomes are abundant in the wing cell layers of the corneal epithelium, adherens junctions throughout the layers, and hemidesmosomes in the basal cell layers. They provide structural integrity and anchoring function of adjacent cells as well as adhesion to the substrate [14]. Gap junctions in the basal cell layers mediate intercellular communication and play an important role in cell differentiation. Sensory nerve fibers that originate and derive from the ophthalmic branch of the trigeminal ganglion enter the cornea peripherally and terminate in numerous fine endings among the epithelial cells. The integrity of nerve fibers is crucial for normal corneal function by sensing thermal, mechanical, and chemical stimuli leading to the release of essential neurotrophins for corneal homeostasis and wound healing [66,67]. In recent years dysregulation of the epithelial barrier function has been recognized as a primary defect in the pathogenesis of allergic reactions [14,68,69]. Allergic forms of OSD are frequently associated with excess tearing and itching sensation. Excess tearing causes an instable tear film with decreased lubricating properties and may thus result in signs similar to DED. Topographical irregularities such as papillae will moreover cause localized elevated friction. As mentioned, eye rubbing as an answer to itching eyes will enhance the inflammatory reaction of the eye. In children suffering from neurodermitis special care should be taken to prevent the development of ocular allergies and keratoconus. Prescribing non-preserved lubricating eye drops to prevent friction, itching and consequently eye rubbing should be taken into consideration. Last but not least the compromised epithelial barrier function leads to elevated susceptibility to allergens [13,70]. An established technique to assess epithelial barrier function is the measurement of fluorescein uptake by the cornea [71-73]. However, there is so far no commercial instrument for routine clinical diagnosis of compromised epithelial barrier function.
Inflammation is an essential regulator of epithelial wound healing. If however the stimulus of irritation persists, it may trigger chronic ocular surface inflammation. Tear film hyperosmolarity has been postulated to be the most prominent cause of self-sustaining ocular surface inflammation in dry eye disease [27,29]. This judgement has recently been questioned [74]. Vazirani emphasizes that other processes like systemic autoimmune disease may be the driving force, and warned against an oversimplification of concepts and extrapolation beyond the available data. Recently Davis and colleagues pointed out that systems immunology is just getting started [75]. For much of its history, most insights into the immune system came from examination of the parts and their largely individual properties, and not so much of activities of the entire system currently comprising 350 differentiation cluster (DC) antigens, over 100 cytokines and chemokines, at least as many cell subsets, and thousands of genes. Coming years will result in the discovery of new relationships and participants in immunology. For the moment, however, a coherent understanding of the quantitative rules that govern cytokine-mediated cell-to-cell communication is still lacking [76]. In this situation it may be wishful thinking to believe that blocking one step of one known pathway of the inflammation process (= defined mode of action of an active ingredient in an eye drop) will break the entire ‘vicious circle’ of chronic ocular surface inflammation. As pointed out by Aya and colleagues with respect to wound healing, it may be worthwhile also to have a closer look into the complex role of high molecular weight hyaluronan in shifting chronically inflamed tissues toward homeostasis [77].
References
- https://www.sciencedirect.com/science/article/abs/pii/S0195666308005114?via%3Dihub
- https://www.sciencedirect.com/science/article/pii/S0098299716300231
- https://muse.jhu.edu/article/405752/pdf
- https://www.sciencedirect.com/science/article/pii/S1542012417301192?via%3Dihub
- https://www.sciencedirect.com/science/article/pii/S1542012417301209?via%3Dihub
- https://www.reviewofoptometry.com/article/why-dry-eye-trials-often-fail
- https://www.researchgate.net/publication/11268464_Ocular_allergy_guidelines_A_practical_treatment_algorithm
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1397720/pdf/bmj33200584.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2852024/pdf/nihms184967.pdf
- https://synapse.koreamed.org/Synapse/Data/PDFData/0166AAIR/aair-10-207.pdf
- https://www.jci.org/articles/view/124608/pdf
- https://bjo.bmj.com/content/bjophthalmol/82/7/797.full.pdf
- https://pubmed.ncbi.nlm.nih.gov/16427347/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3858173/pdf/nihms533464.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1041892/pdf/brjopthal00602-0046.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1723585/pdf/v084p00834.pdf
- https://onlinelibrary.wiley.com/doi/epdf/10.1111/cxo.12038
- https://pubmed.ncbi.nlm.nih.gov/28601391/
- https://www.researchgate.net/profile/Alejandro_Tello2/publication/309183993_Risk_Factors_for_Keratoconus_Atopy_and_Eye_Rubbing/links/5c3333cc92851c22a361607f/Risk-Factors-for-Keratoconus-Atopy-and-Eye-Rubbing.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3816990/pdf/mv-v19-2124.pdf
- http://scholar.google.de/scholar_url?url=http://ojs.ptbioch.edu.pl/index.php/abp/article/download/1923/556&hl=de&sa=X&scisig=AAGBfm04Wekha1QcYHxsjI7ox9lVZ7Z4jw&nossl=1&oi=scholarr
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4506344/pdf/eye201563a.pdf
- https://iovs.arvojournals.org/article.aspx?articleid=2212828
- http://downloads.hindawi.com/journals/dm/2016/1243819.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5392225/pdf/mehdiophth-6-001.pdf
- https://pubmed.ncbi.nlm.nih.gov/15590483/
- https://www.sciencedirect.com/science/article/pii/S1542012417301349?via%3Dihub
- https://pubmed.ncbi.nlm.nih.gov/475632/
- https://www.sciencedirect.com/science/article/pii/S1542012413000906?via%3Dihub
- https://bjo.bmj.com/content/bjophthalmol/100/3/300.full.pdf
- https://www.researchgate.net/publication/332046680_A_gut-to-brain_signal_of_fluid_osmolarity_controls_thirst_satiation
- https://pubmed.ncbi.nlm.nih.gov/24785271/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4455064/pdf/ncomms8150.pdf
- https://pubmed.ncbi.nlm.nih.gov/29620566/
- https://www.karger.com/Article/FullText/501712
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3259007/pdf/z7g2418.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3720150/pdf/i1552-5783-54-7-4900.pdf
- https://pubmed.ncbi.nlm.nih.gov/24959988/
- https://www.sciencedirect.com/science/article/pii/S001448351500144X?via%3Dihub
- https://bmjophth.bmj.com/content/bmjophth/3/1/e000146.full.pdf
- https://www.sciencedirect.com/science/article/pii/S0181551219300555?via%3Dihub
- https://www.sciencedirect.com/science/article/pii/S0014483501910450?via%3Dihub
- https://www.sciencedirect.com/science/article/abs/pii/S0074769603310010?via%3Dihub
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3889467/pdf/nihms533449.pdf
- https://www.springer.com/de/book/9783662159392
- Kaura, R. and J.M. Tiffany, The Role of Mucous Glycoproteins in the Tear Film, in The Preocular Tear Film in Health, Disease, and Contact Lens Wear, F.J. Holly, D.W. MLamberts, and D.L. MacKeen, Editors. 1986, Dry Eye Institute: Lubbock, TX. p. 728-731.
- https://pubmed.ncbi.nlm.nih.gov/1778667/
- Tiffany, J.M., Viscoelastic properties of human tears and polymer solutions. Adv Exp Med Biol, 1994. 350: p. 267-70.
- Tiffany, J.M., J.C. Pandit, and A.J. Bron, Soluble Mucin and the Physical Properties of Tears, in Lacrimal Gland, Tear Film, and Dry Eye Syndromes 2, D.A. Sullivan, D.A. Dartt, and M.A. Meneray, Editors. 1998, Springer, Boston: Boston, MA. p. 229-234.
- https://link.springer.com/chapter/10.1007/BFb0050985
- https://onlinelibrary.wiley.com/doi/abs/10.1002/pol.1981.130190507
- https://iovs.arvojournals.org/article.aspx?articleid=2158516
- https://iovs.arvojournals.org/article.aspx?articleid=2200174
- https://pubmed.ncbi.nlm.nih.gov/17177679/
- https://pubmed.ncbi.nlm.nih.gov/18782111/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2666617/pdf/nihms97876.pdf
- https://www.sciencedirect.com/science/article/pii/S1350946218300624?via%3Dihub
- https://www.scirp.org/pdf/OJOph_2017071415134633.pdf
- https://www.nature.com/articles/s41598-018-30002-x.pdf
- https://pubmed.ncbi.nlm.nih.gov/8563428/
- https://pubmed.ncbi.nlm.nih.gov/20168216/
- https://eye-cataract-surgery.imedpub.com/sandbank-epitheliopathy-of-the-conjunctiva-sec-a-new-indicator-in-dry-eye-diagnostics-useful-for-optimized-ocular-surgery.pdf
- https://www.oatext.com/pdf/NFO-4-199.pdf
- https://pubmed.ncbi.nlm.nih.gov/30783943/
- https://www.nature.com/articles/6700617.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4651844/pdf/nihms-709418.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5706540/pdf/nihms920837.pdf
- https://ctajournal.biomedcentral.com/track/pdf/10.1186/2045-7022-1-5
- https://www.jacionline.org/article/S0091-6749(11)00864-5/pdf
- https://www.surveyophthalmol.com/article/S0039-6257(18)30152-8/fulltext
- https://pubmed.ncbi.nlm.nih.gov/1914501/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1722655/pdf/v082p00797.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6007839/pdf/pone.0198831.pdf
- http://www.ijo.in/temp/IndianJOphthalmol66144-7204433_200044.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5790187/pdf/nihms936962.pdf
- https://www.nature.com/articles/s41577-019-0131-x
- https://onlinelibrary.wiley.com/doi/pdf/10.1111/wrr.12214